| Novolin U | |
|---|---|
| Ultralente by Novo Nordisk | |
| long-acting | |
| r-DNA/GE/GM | |
| U40, U100 | Zinc |
Action in dogs:
| |
| Line: Novolin | |
| Also known as: Ultratard | |
| Similar to: Huminsulin Ultralong, Humulin U Humulin UL, Humulin Ultralenta Humulin Ultralente, Humulin Zn Humuline Ultralong, Humulina Ultralenta, Umuline Zinc Names of Lilly r-DNA/GE/GM insulins worldwide | |
| Use and Handling: | |
| Shelf Life: 30 months | Type: cloudy |
| When Opened: 6 weeks room temp. | |
| In Pen: N/A | |
| Notes: Protect from light and heat Do Not Freeze, Resuspend Do not use if product does not re-suspend Do not use intravenously [1] store at 2-8C | |
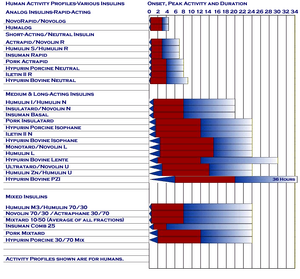
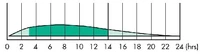
Time activity profile for Novolin U.
The name for Novo Nordisk's Ultralente insulin sold in North America. It was a U100, lente-type, long-acting insulin, now discontinued.
Novo's Euro name for the same insulin was Ultratard. [2][3] Both were crystalline-suspended insulins. [4]
It is comparable to Humulin U, [5] Umuline Zinc, Humulina Ultralenta, Humuline Ultralong, and Humulin Zn. [6][7]
What Ultralente Is Not[]
No Lente-type insulin regardless of species can contain any NPH/isophane insulin [8] or any R/Neutral insulin. [9][10]
Both are chemically impossible: the phenol preservative present in NPH/isophane alters the action of Lente-type insulins, creating a mixture with an approximate action of R/Neutral. [11][12]
The zinc suspension of Lente-type insulin binds R/Neutral, causing the short-acting insulin to slow, losing its short-acting effect. [13][14]
Before the invention of VetPen, Lente-type insulins could not be dispensed in pen or cartridge form because the glass ball formerly used to mix the insulin in these devices shattered the Lente crystals.[15]
Combining Lente Family Insulins[]
Insulin manufacturers [16] indicate that R/neutral and semilente, Lente, ultralente insulins are able to be combined in the same syringe, but only just before injection. In pre-filled syringes, the zinc suspension of the Lente-type insulins binds the R/neutral, causing it to lose its short-acting effect. Various studies have documented this, and some doctors advise against using R/neutral in the same syringe with the Lente family of insulins. [10][17][18][19]
|
None of the Lente family of insulins (semilente, Lente, Ultralente) can be combined with [11] NPH/isophane insulins. The phenol preservatives present in NPH-type insulins alters the Lente-types to the point where they become a close approximation of R/neutral, with regard to action. [19] Keeping the phenol preservatives in mind, all protamine-suspended insulin mixes would be "off limits" regarding same syringe mixing with any Lente-type insulins. [19] | ||
|
Ultralente Insulins | |
|---|---|
|
Long acting Non-soluble |
| Humulin U, Humulin Zn, Humulina Ultralenta Humuline Ultralong, Umuline Zinc Humulin UL, Huminsulin Ultralong (No longer Produced.) Novolin U, Ultratard Humulin Ultralenta, Humulin Ultralente (No longer produced.) | |
| Iletin Ultralente [20] (No longer produced.) Ultratard [21] (No longer produced.) | |
| Iletin I Ultralente (No longer produced.) | |
The Novo insulins,Ultratard and Monotard, were available slightly longer in Europe. [3]
From the announcement: Monotard and Ultratard will not be available in the UK after February 2006. The discontinuation of these insulins was initially announced in September 2004. Initially the discontinuation was scheduled for February 2006, however this date has now been brought forward to October 2005.
References[]
- ↑ Maddison, Jill E.,Page, Stephen W.,Church, David B. (2008). Small Animal Clinical Pharmacology. Saunders Ltd..
- ↑ Prescribing Novo Insulins.
- ↑ 3.0 3.1 Ultratard/Monotard Discontinuation. Diabetes UK (July 2005). Cite error: Invalid
<ref>tag; name "Monotard" defined multiple times with different content - ↑ Scientific Discussion-Ultratard. EMEA.
- ↑ Humulin U Discontinuation Notice. US Food and Drug Administration (July 2005).
- ↑ Discontinuation of Humulin Zn. Diabetes UK (July 2004).
- ↑ Lilly EU/UK Product Sheet & Time Activity Profiles. Eli Lilly.
- ↑ Combining Lente-type Insulins with Phenol-Preserved Insulins. National Federation for the Blind.
- ↑ Lente Zinc Suspension Causes Loss Of R/Neutral Short-Acting Effect. Endotext.org.
- ↑ 10.0 10.1 Huffman DM, Garber AJ. (1991). Availability of Soluble (R/Neutral) Insulin in Mixed Preparations With Crystalline (Lente) & Ultralente GE Insulin. Clinical Therapeutics. Cite error: Invalid
<ref>tag; name "Huffman" defined multiple times with different content - ↑ 11.0 11.1 Lente-Type Insulins & NPH/Isophane Insulins-A Bad Combination. National Federation for the Blind. Cite error: Invalid
<ref>tag; name "Bad" defined multiple times with different content - ↑ Havlik I, Galasko G, Alberts E, Furman KI, Seftel HC. (1988). Solubility Changes on Mixing Short- and Long-acting Insulin Preparations. South African Medical Journal.
- ↑ Deckert T. (1980). Intermediate-Acting Insulin Preparations: NPH (Isophane) & Lente. Diabetes Care.
Note--in 1980, there were no r-DNA/GE/GM insulins - ↑ Resource Guide. American Diabetes Association (2005).
- ↑ Hanas, Ragnar (1999). Insulin-Dependent Diabetes--Page 10. Children With Diabetes.
- ↑ Insulin Producers vs Doctors Re:Combining R/Neutral & Lente-type Insulins. Endotext.org.
- ↑ Bilo HJ, Heine RJ, Sikkenk AC, van der Meer J, van der Veen EA. (1987). Absorption Kinetics & Action Profiles-Single Subcutaneous Administration of Human Soluble (R/Neutral) & Lente Insulin. Diabetes Care.
- ↑ Heine RJ, Sikkenk AC, Eizenga WH, van der Veen EA. (1983). Delayed Onset of Action of Soluble (R/Neutral) Insulin After Premixing With Lente Insulin. Diabetes Research & Clinical Practice.
- ↑ 19.0 19.1 19.2 Insulin Therapy-Mixing Precautions. RxEd.org.
- ↑ Discussion of differences between r-DNA Ultralente and beef Ultralente insulins. Free Patents Online.
- ↑ Insulin Preparations. Committee on Safety in Medicines-UK (1976).
More Information[]
- NACDS-Insulin Chart-Page 2
- Insulin therapy for dogs and cats Dowling, Patricia, September 1995, Canadian Veterinary Journal
A discussion of Ultralente insulins.
- Lente Insulins-Injectable Suspensions West Virginia University College of Pharmacy-2009
- Insulin human zinc, extended (Ultralente)
 |
 |
 | |